[ad_1]
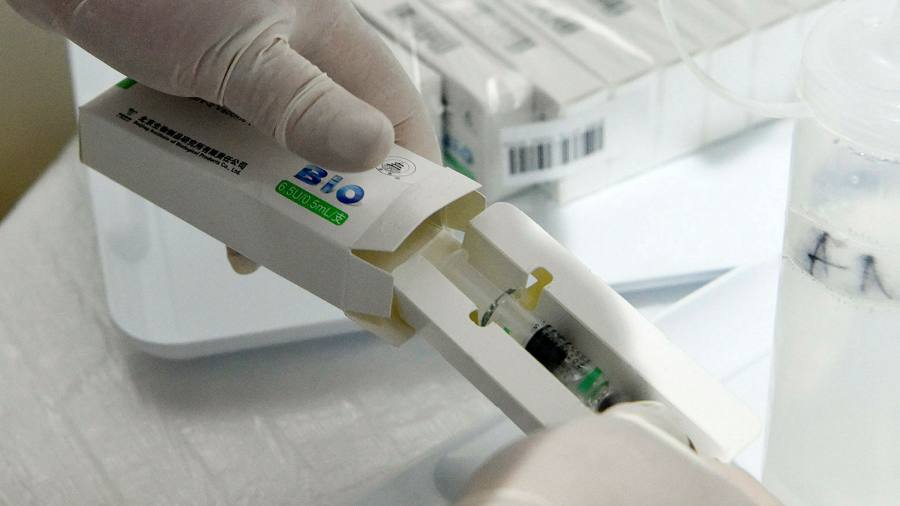
The World Health Organization has added a Sinopharm vaccine to its list of approved applications of Covid-19, raising the credentials of the dose made in China amid doubts about its effectiveness.
The emergency use list for the shot, developed in collaboration with the Beijing Institute of Biological Products, is for a two-dose regimen for all adults over the age of 18, Tedros Adhanom Ghebreyesus said. , Director-General of the WHO.
It is the first vaccine of any kind made in China to receive WHO emergency use authorization. Approval is a signal to countries that the Vaccine against Sinopharm it is safe to use and means the punch will be added to the WHO Covax vaccine acquisition program.
Alejandro Cravioto, chairman of the WHO’s strategic advisory group of experts on immunization, said the group had made a “thorough assessment” of the vaccine and that there was “sufficient evidence” that it was safe and reduced cases of severe or symptomatic disease at least 79 percent.
Initial vaccine studies were conducted primarily in China, where the coronavirus has already been contained. The final phase, or phase 3, was conducted in other countries. Sinopharm has a second two-dose coronavirus vaccine, which it developed with the Wuhan Institute of Biological Products, which has not yet been approved by the WHO.
WHO authorization is a victory for Sinopharm, which is the dominant producer of vaccines for China’s domestic market and supplies most of the spikes for state vaccination programs, but has not yet been established as a vaccine exporter.
The company did not immediately respond to a request for comment.
China has promised Covid vaccines worldwide in development, but international deployment has been hampered by persistent concerns about their effectiveness, as Chinese manufacturers have been slower than Western candidates to publish detailed trial data .
The WHO noted a relative lack of data in elderly patients, and recommended that surveillance studies be conducted in countries where prey is administered to elderly people.
The approval of the spike means it could be used in countries suffering from devastating Covid waves, such as India and Brazil, the WHO said.
Mohga Kamal-Yanni, a health policy expert for the People’s Vaccine Alliance, said the approval was “great news for people in developing countries who have been watching the vaccination of people from rich countries while they have stayed in the back of the queue ”.
Mariângela Simão, WHO Deputy Director-General for Access to Medicines, said the approval had the potential to “quickly” accelerate access for countries that want to protect health workers and those most at risk. .
[ad_2]
Source link