[ad_1]
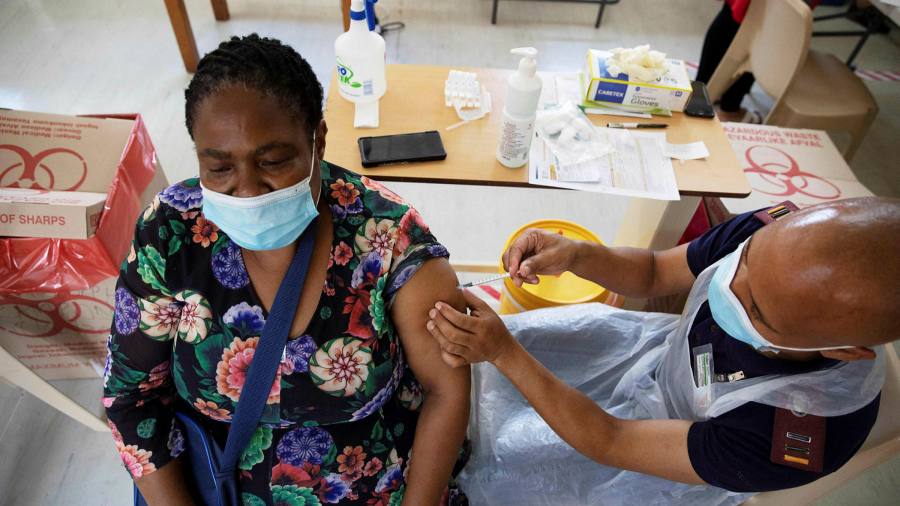
The South African health regulator has recommended resuming the use of the Johnson & Johnson coronavirus vaccine, days after the country followed the United States with a break to assess the risk of blood clotting with the single dose.
The lifting of the break should be conditional on “enhanced control and monitoring of participants who are at high risk for blood clotting disorder” and updated plans to treat any vaccine-related clots, the chief said. week the South African Health Products Regulatory Authority. Expert ethics committees must also approve before the country can restart.
The J&J vaccine was the only one deployed in South Africa, until the country suspended it on Tuesday and risked causing further vaccinations in Africa’s most industrial economy.
The U.S. Centers for Disease Control and Prevention and the U.S. Food and Drug Administration recommended an indefinite suspension after six cases of a rare blood clotting disorder of nearly 7 million doses administered to USA. Use of the J&J vaccine in the United States continues to be suspended after experts said this week that they did not have enough evidence to change their stance.
No cases of blood clotting have been reported among the nearly 300,000 vaccinated health workers in South Africa before the suspension.
“The reason it’s important to recognize this extremely rare side effect is that treatment differs from the usual way clots are treated, and these serious complications could be mitigated with quick and effective treatments,” the council said this week. of South African Medical Research.
The government of President Cyril Ramaphosa was under pressure to speed up the slow pace of vaccinations even before the US pause interrupted its plans.
So far, South Africa has recorded more than 1.5 million cases, the African country most affected by the pandemic, especially after an intense second wave during the new year. Since then, the daily count of new cases has dropped markedly, but there are fears that there will be a third wave around the corner.
South Africa switched to J&J at the last minute in February, following an original plan to use the Oxford / AstraZeneca vaccine that led to evidence that it does not protect against milder diseases in cases of a variant that is has become dominant in the country.
South Africa’s supplies of the J&J vaccine have been limited to date, as initial deployment among health workers resulted in a research study rather than commercially ordered deliveries.
The largest J&J deliveries are due later this month and will be used in the second phase of the launch, prioritizing elderly and vulnerable South Africans.
The Ramaphosa government has ordered 31 million J&J shots and 30 million two-dose Pfizer vaccines to reach its goal of vaccinating 40 million people. Online registration for those over 60 began on Friday.
This week, the main opposition of the Democratic Alliance said that, unlike South Africa, the United States had the “luxury” of suspending the J&J vaccine, as millions of Americans have already received shots from others. drug manufacturers.
“Stopping the administration of a vaccine does not affect them as much as it could affect South Africa, as we currently only have small amounts of this unique vaccine available,” the party said.
[ad_2]
Source link