[ad_1]
If a national hydrogen economy is to exist, we need to change a few things. One of them is how we produce hydrogen, most of which comes from fossil fuels. SunGreenH2 brings a nanotech-level advance to green hydrogen production, using high-energy electrolysis so orders of magnitude more hydrogen can be made cleanly directly from water.
Hydrogen is used everywhere, but the lack of scalable and green production options has slowed its adoption. What’s the point of having a hydrogen battery system for renewables if you have to generate that hydrogen from natural gas, oil, and coal?
The answer is the process of electrolysis, which splits water molecules into atoms and produces hydrogen and oxygen. Sounds great, but manufacturing the catalysts and coatings that make up the technological sandwich of any modern electrolyzer cell requires a lot of energy and expensive materials like platinum. Therefore, green hydrogen is many times more expensive than dirty hydrogen, and it is difficult to produce.
Here comes SunGreenH2, a major improvement on a critical part of those cells: the electrode. The company says it will double hydrogen production, lowering its cost and reducing its reliance on platinum and other rare elements at the same time.
“We are developing a proprietary alloy-based cathode that doubles the surface area available for reaction,” said CEO and co-founder Tulika Raj. “This doubles the amount of hydrogen that can be produced there. But that’s not enough, you have to be scalable and produceable. So it doesn’t use precious metals – in this case we’re reducing the amount needed by 30x.”
She said that practically all modern electrolyzers can use this improved electrode. “The idea is to abandon the entire industry,” she said.
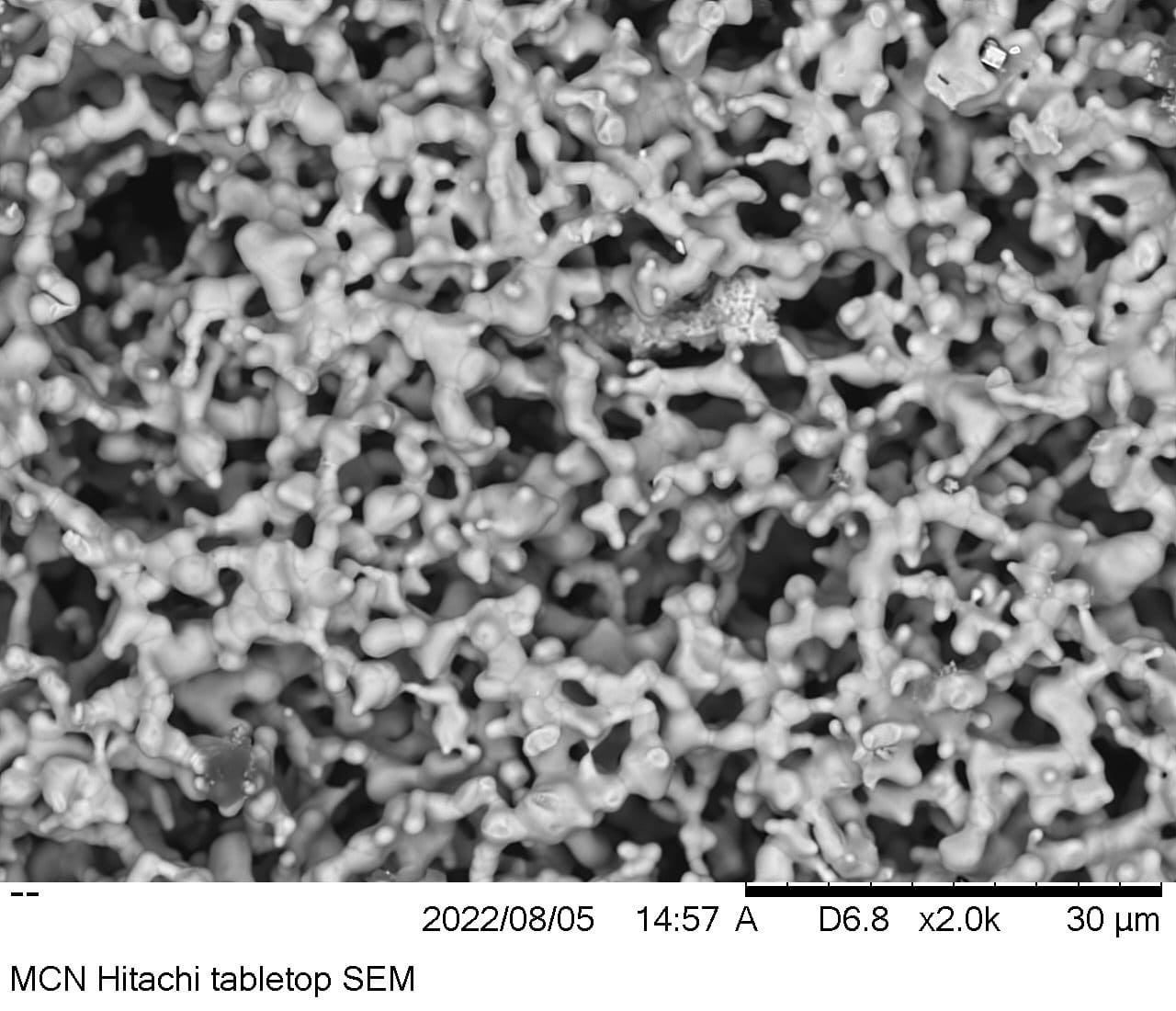
The nanostructure surface is the first breakthrough, and you can see a scanning electron microscope image here.
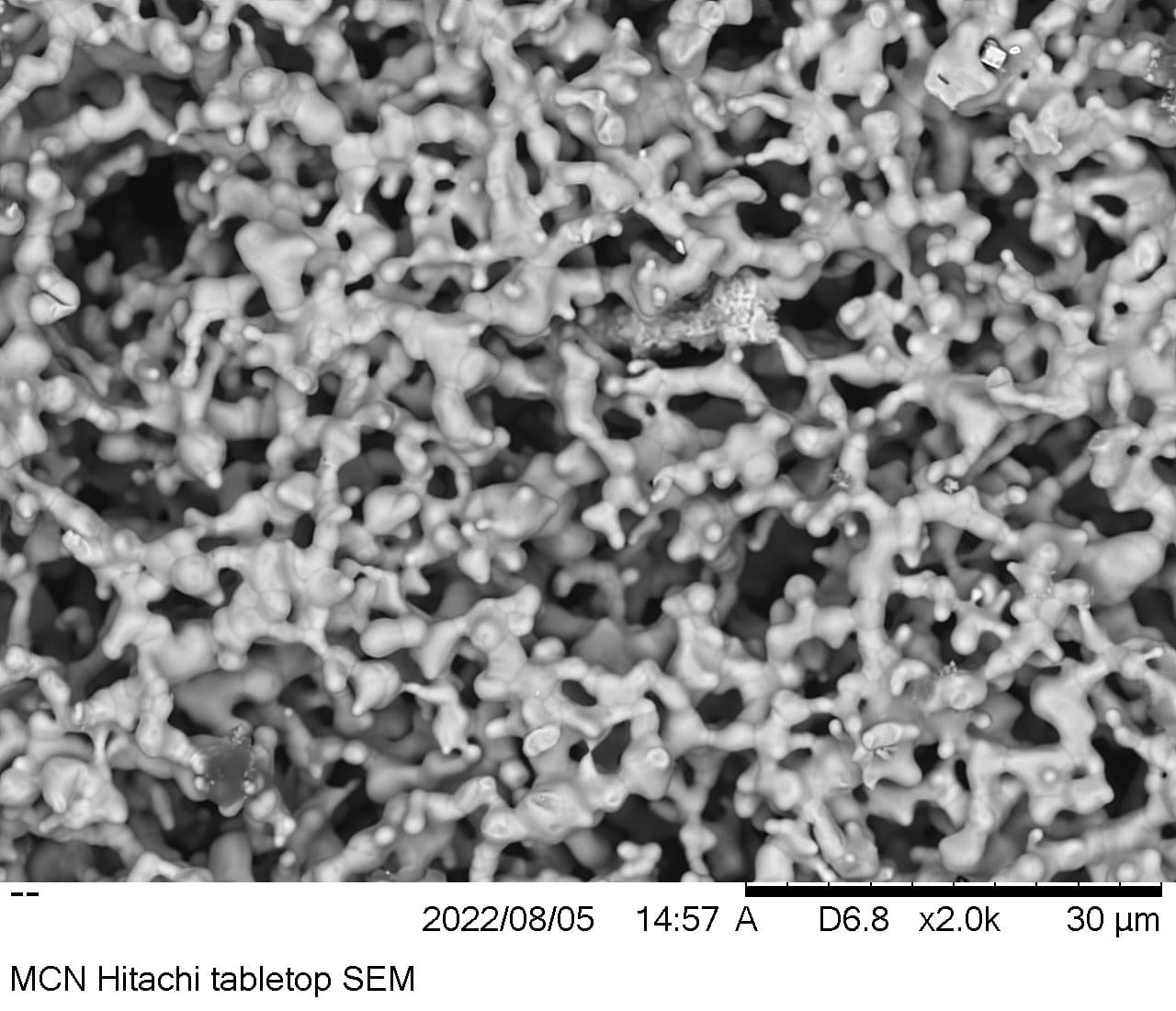
Image Credits: SunGreenH2
It sounds a bit messy, but the material is carefully designed to interact with other chemical components in the electrolysis process (especially water and catalyst) and doubles as a 3D sponge structure instead of a flat or rough one. The surface area where a reaction can occur.
One of the weaknesses of nanostructures is that they are very weak and prone to decay, so they reduce their effectiveness (however miraculous) over time. SunGreenH2 gets ahead of this shortcoming with the “Sacrifice Trigger Concept”.
“Increasing the surface area and adjusting the crystallinity of the deposited material leads to an improvement in performance and stability, respectively, using the sacrificial catalyst to significantly increase the lifetime of the core,” explained Raj. “In the current/current technology, due to the corrosion of the catalyst, the performance of the electrode decreases. In the technology developed by SunGreenH2, the sacrificial catalyst corrosion causes an increase in the surface area, which compensates for the corrosion effect.
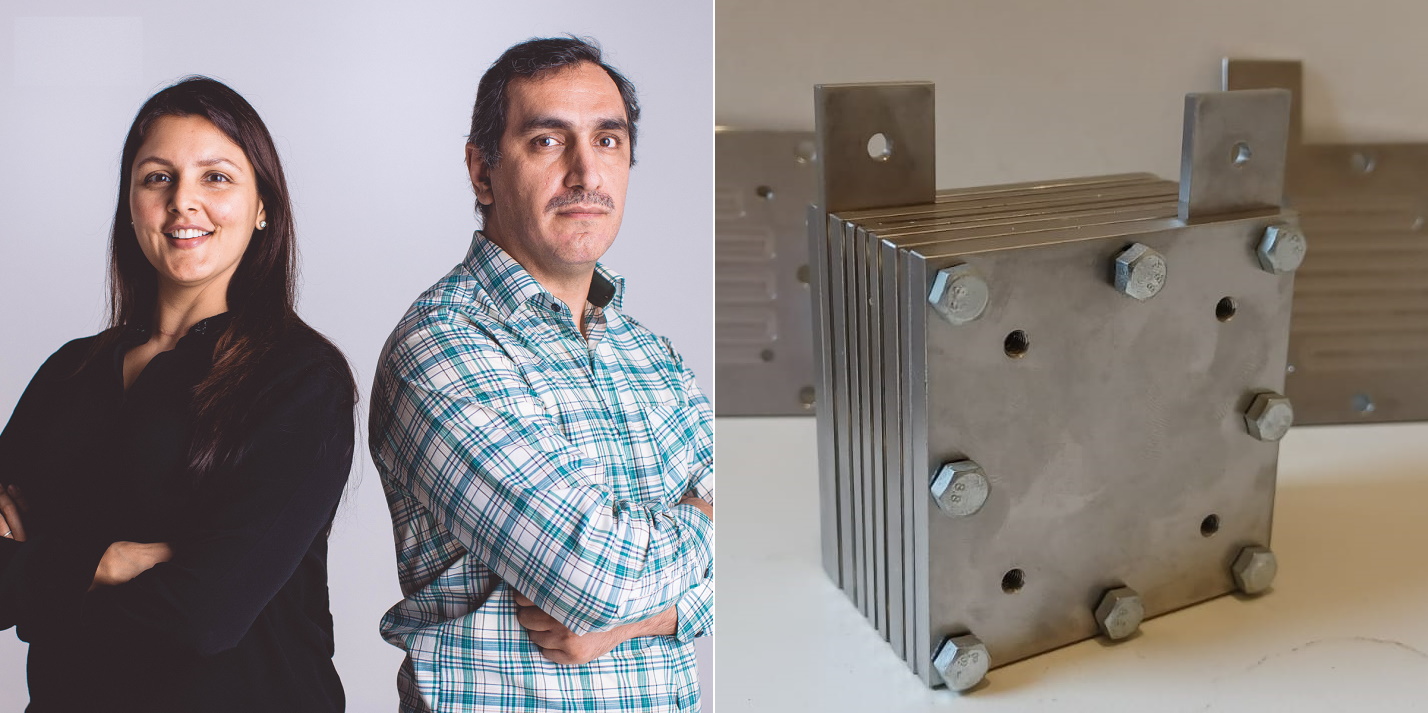
SunGreenH2 founders Tulika Raj (left) and Saeed Masudi Panah. On the right, a prototype electrolysis stack with the company’s technology somewhere.
In other words, this secondary material fills the structure, and the corrosion process (in some way beyond my understanding) makes the original catalytic surface hot.
The company has raised a $2 million seed round led by SGInnovate with participation Vinci BV, Cap Vista, Entrepreneur First, SOSV HAX, she1K and Apsara Investments. According to Raj, this money will be used to set up manufacturing facilities in Melbourne for the first time to meet the needs of their partners, confidential but includes major energy concerns in the European Union, USA, Canada, Japan and Singapore.
Although it initially aims to sell electrolysis components independently, it plans to partner with system integrators next year to produce full electrolysis stacks and eventually produce end-to-end solutions with larger companies. “We also apply our platform technology to produce similar green transition materials, such as electrodes, PTLs and bipolar plates for fuel cells, electrodes for batteries and photo electrodes for direct sunlight to hydrogen panels,” he said.
It all depends on their success in these early stages, but if the green hydrogen revolution is to happen, the industry must start adopting such technologies as soon as possible.
[ad_2]
Source link