[ad_1]

TherMedical announced today that it has received FDA approval for a clinical trial to evaluate its SERF removal system.
Waltham, Massachusetts-based Thermedical will conduct an open-label, single-arm interventional clinical trial to evaluate the safety and efficacy of the Thermedical SERF ablation system with the Durablet catheter.
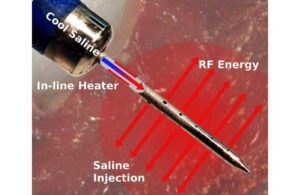
TherMedical has designed the SERF ablation system with a Durablet catheter to treat patients with ventricular arrhythmias with antiarrhythmic drugs or standard ablation procedures. Current treatment for VT comes in the form of implantable cardiac defibrillators (ICDs). Patients with ICDs who experience VT episodes can be treated with conventional radiofrequency ablation.
According to News, the system features a new biological heat transfer system designed to be more efficient than conventional ablation methods. The tissue in the heart wall where VT is the cause of life-threatening arrhythmias.
“We are grateful to the FDA for the rapid approval of this critical trial to evaluate our Saline-Enhanced Radiofrequency (SERF) Ablation System for patients who have run out of treatment options for their VT episodes and suffer from a very poor quality of life,” Michael Curley, TherMedical’s founder and CEO, said in the release. “In our recent multicenter trial, 31 out of 32 participants experienced immediate elimination of clinical VT at the end of the procedure, and treatments such as shock or pacemaker were reduced by 89% within five months. Patients.”
The Mayo Clinic (Rochester, Minnesota) will be the first of about 25 trial sites in North America, with cardiac electrophysiologist Dr. Douglas Packer as the principal investigator.
Additional sites are planned for Birmingham, Alabama, Boston, Charleston, South Carolina, Montreal, Nashville, Tennessee, Philadelphia and Quebec City. The trial will enroll 154 participants with recurrent, persistent, monomorphic VT resistant to drug therapy and routine catheter ablation.
Participants will undergo SERF elimination with follow-up at seven days, one month, three months, and six months.
“This large clinical trial is critical to evaluating this new approach to treating problematic VT to reduce or eliminate shocks from implantable cardioverter defibrillators in this patient population,” Curley said. “We are encouraged by the initial data and are eager to further demonstrate the safety and efficacy of the promising new SERF technology.
“In addition, SERF offers hope to people suffering from this condition, as it is a life-changing treatment for refractory VT.”
[ad_2]
Source link