[ad_1]
Conferences in exotic locations, scientific discussions lasting a few hours to a four-day family holiday package, expensive gadgets and gold coins as gifts and cash in bank accounts – the extent of medical corruption in India is visible to all without anyone realizing it. it is.
Threats continue to grow. The infamous 40 per cent commission – allegedly demanded by government contractors in Karnataka – seems to have made its way into the medical field as well.
“The commission given to doctors (for prescribing certain drugs) has increased from 20 percent earlier to about 40 percent. Sharan*, a medical and sales representative with 22 years of experience, says many top pharma companies offer such discounts and the money is kept in the accounts of the doctors’ relatives. .
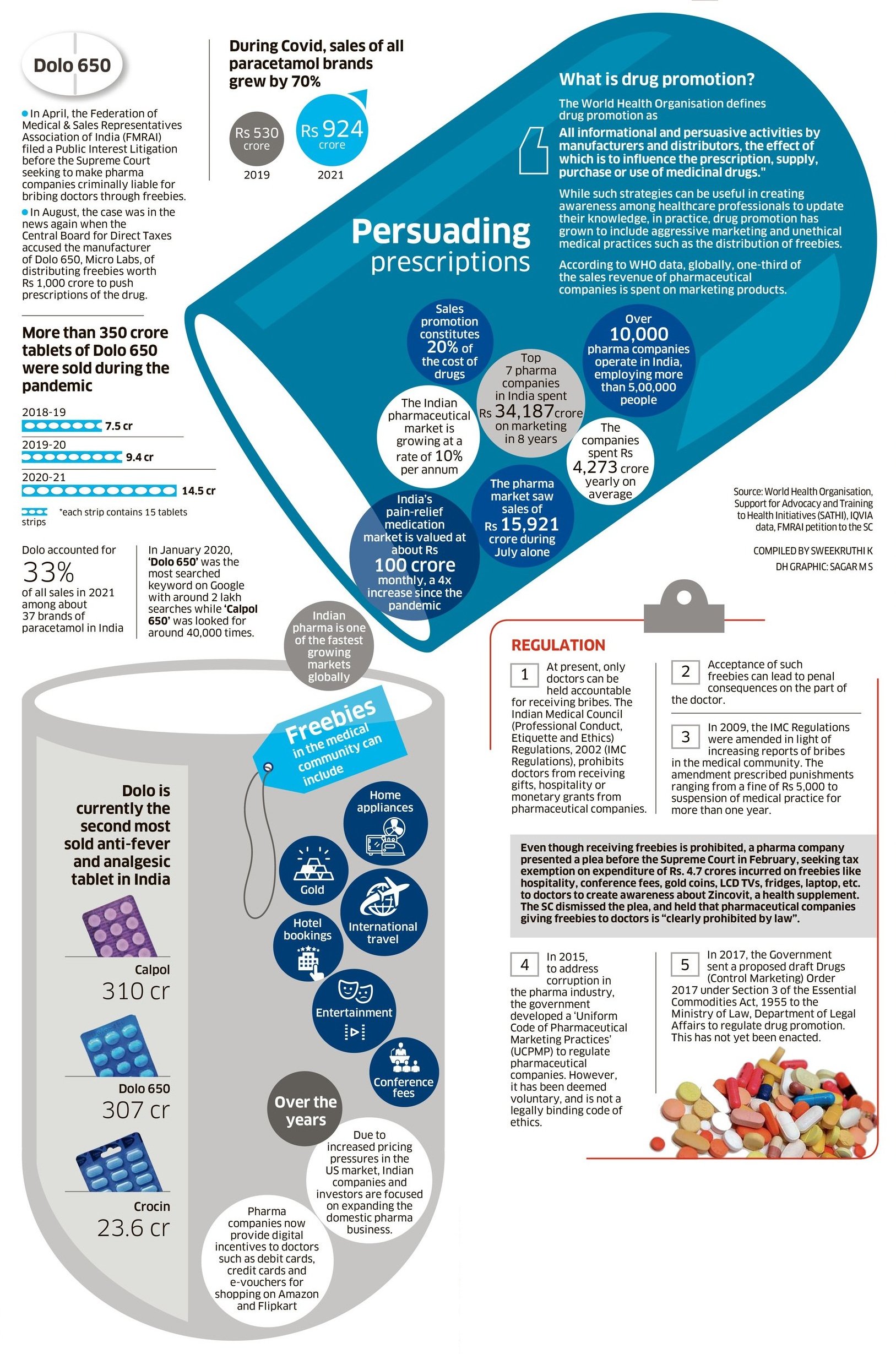
Not all doctors are corrupt and not all pharmaceutical companies use unethical methods to sell their products. However, it cannot be denied that a large number of doctors and medical companies use unfair methods. Even the Supreme Court was outraged at the cost of promoting Dolo 650, a popular brand of paracetamol, during the pandemic.
A Supreme Court bench of Justice D Chandrachud and Justice AS Bopana, hearing a petition on medical corruption last month, described it as a “serious case” when it was revealed that the manufacturers of Dolo-650 had spent nearly Rs 1,000 crore for free. For doctors, according to the Central Board of Direct Taxes. “This is not music to ears. Even I was asked to take the same medicine when I had Covid,” Justice Chandrachud said.
The bench was hearing a petition filed by the Association of Medical and Sales Representatives of India (FMRI), which sought legalization of the Uniform Pharmaceutical Marketing Practices (UCPMP). They also called for a monitoring mechanism to increase transparency, accountability and ensure consequences for violations.

Pending such a regulation, the federation requested the apex court to “issue guidelines to regulate and regulate unethical marketing practices by pharmaceutical companies or alternatively maintain the code with such amendments/additions as the Supreme Court finds valid and reasonable.” He said.
FMRAI In India, pharmaceutical companies have spent huge amounts of money on sales promotion to influence doctors to prescribe their products to increase drug sales.
“A study has revealed that the seven major companies have together spent Rs 34,186.95 crore on marketing drugs over eight years. Sales promotion costs account for 20 per cent of the cost of drugs, thus taking them far beyond the reach of the common man,” said FMRAI’s petition to the Supreme Court.
Although referred to as ‘sales promotion’, these freebies include direct or indirect benefits to doctors such as gifts and entertainment, sponsored foreign trips, hospitality and other benefits. “Medical representatives promote products to doctors and senior sales managers negotiate with them by offering cars, facilities, foreign trips, etc.,” says Sharan.
There are two broad categories in which the link between medicine and marketing continues.
The first is overuse or overprescription. This can happen in three ways – prescribing more than necessary, prescribing for longer than necessary and prescribing more than necessary.
The second category includes prescribing unreasonable amounts of medication. Such drug combinations, prescribed due to high-pressure promotional strategies, often lack the support of medical literature and may harm health.
Hundreds of these combinations have been banned by the government at various times, but pharmaceutical companies continue to flood the market with them.
A study published in WHO Bulletin found that of 395 fixed-dose combination (FDCs) antibiotics sold across the country, 229 (58 percent) were on the WHO’s “not recommended” list. Of the 20 top selling antibiotic cocktails in India, 13 are not evidence-supported or recommended by the WHO.
Pressure on medical representatives
On the other hand, medical representatives are expected to meet sky-high targets. “If these targets are not met, we will be sacked. Since the targets are high, local administrators (who are in charge of many districts) work continuously in every district and also give concessions to doctors,” said Sharan.
For a Coimbatore-based doctor, the pressure he faces from medical representatives has increased manifold in the last 20 years. “I personally know doctors who have banned medical representatives. When I started practice, there was only one representative per company. Today, there is one representative for each product,” she said.
She noted that over the years, the marketing progress has been increasing. “Most companies hold conferences and invite doctors along with their families. The conference lasts only a few hours, but a two-day visit is planned.
The purpose of drug representatives meeting with physicians was initially to keep medical professionals up-to-date on the latest developments in medicine and treatment. It is especially important for doctors in private clinics to meet sales staff to know the cost of drugs and prescribe affordable options for their patients.
The conferences organized by the companies argue for help professionals to continue medical education.
Critics argue that the information doctors learn from these conferences is one-sided because it is sponsored by the pharmaceutical companies.
Prasana Saligram, a public health researcher formerly associated with the Public Health Foundation of India, said: “Very expensive drugs are being sold as cheap drugs because the patient is only a casual buyer and the product is sold to doctors.
Does the government not know that such medical malpractice exists? What is being done to solve the problem? The Department of Pharmaceuticals under the Union Ministry of Chemicals and Fertilizers has repeatedly informed Parliament that it has no plans to make the UCPMP mandatory.
Rules
In the year The code, which came into effect in 2015, does not give the department any power to resolve complaints of misconduct. Any complaint against a pharmaceutical company should be handled through the Pharmaceutical Marketing Practices (ECPMP) established within each pharmaceutical association. The Ministry held a few meetings with industry associations on implementation, but no further follow-up or action was taken.
However, thanks to a somewhat increased level of awareness, some companies have changed their marketing strategies. “In the past, many companies offered foreign trips, or sent goods to doctors’ offices. It was common. But the National Medical Commission (NMC) has been stricter in the last five or six years. Now companies offer something that helps one. A doctor’s practice or The clinic,” said a Bengaluru-based doctor.
A common practice is to sponsor scientific conferences that adhere to NMC guidelines. Pharmaceutical firms sponsor these events and provide information about their products by displaying stalls outside the hall.
However, in specialties like oncology and cardiology, new drugs are expensive, and marketing is a savior. Large corporations invest in research and development and aggressively push their products.
Companies now sponsor panel discussions where a doctor presents scientific information related to a new drug. These presentations do not advertise the drug directly, the doctor talks about the composition. Outside the hall, organizations display these products. “The doctor who provides the information gets an incentive, which can vary between Rs 10,000 to Rs 1,000 for an hour session, depending on the company and the profile of the doctor,” said the doctor.
In February, the Supreme Court, in another decision, made it clear that the practice of pharmaceutical companies granting exemptions to doctors is “clearly prohibited by law.” A bench comprising Justices UU Lalit and S Ravindra Bhatt ruled that medical professionals are prohibited from accepting such gifts or gratuities.
“It is a matter of public importance and concern when it is confirmed that prescriptions can be prescribed and gifts such as gold coins, refrigerators and the like are encouraged to use the freebies provided by pharmaceutical companies. LCD televisions for vacations or medical conferences to fund international trips,” the bench explained.
According to a drug inspector in Thiruvananthapuram, despite such observations, the menace continues to intensify as there is no effective control of doctors who prescribe any particular brand of medicine. Also, there is no provision to take legal action against any doctor for prescribing any particular brand.
In the year In 2017, the Union Government proposed a new regulation, and the draft of the Drugs (Controlled Transaction) Order, 2017 was sent to the Law Ministry. “However, for reasons well known to the Ministry of Law, the same law was not issued. Therefore, the UCPMP that exists today remains voluntary in nature, thus making its implementation impossible,” FMRI summarized.
(With inputs from Mirthyunjay Bose in Mumbai, ETB Sivapriya in Chennai and Arjun Raghunath in Thiruvananthapuram)
(*Some names have been changed upon request)
[ad_2]
Source link