[ad_1]
The president called on the FDA to make hearing aids available over the counter last year in his Promoting Competition in the American Economy executive order to lower costs and increase competition in certain industries.
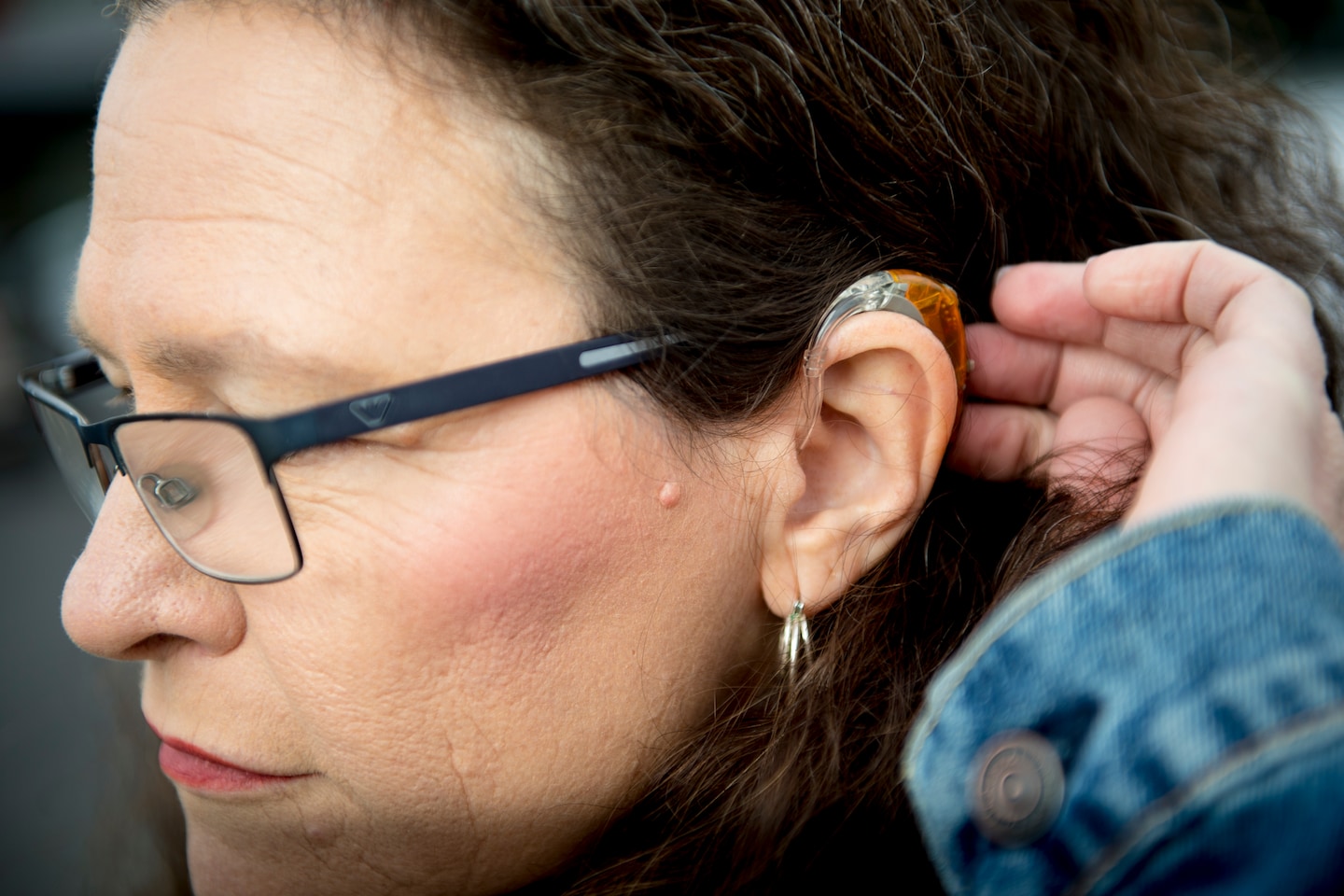
The new regulations will create a new category of hearing aids that supersede state-level regulations requiring patients to visit physicians or audiologists to get prescriptions and fittings. The devices will be available for individuals 18 and older with mild to moderate hearing loss at pharmacies, stores and online.
Sen. Elizabeth Warren (D-Mass.), a co-sponsor of the Over-the-Counter Hearing Aid Act, praised the decision on Twitter and credited Biden with moving the issue forward after it stalled at FDA.
“It took years of hard work, but I’m glad that millions of Americans — many of whom aren’t using hearing aids because they are too expensive — will soon be able to buy safe & affordable hearing aids over the counter,” she tweeted. “This is what it looks like when government works for working people.”
The change is expected to significantly benefit older adults — individuals who are most likely to experience hearing loss and to be on a fixed income — as well as those in poor and rural communities that have fewer audiologists.
The move comes more than four years after Congress ordered the FDA to craft regulations for over-the-counter devices.
“This rule is expected to help us achieve quality, affordable health care access for millions of Americans in need,” said Health and Human Services Secretary Xavier Becerra. “Today’s action by the FDA represents a significant milestone in making hearing aids more cost-effective and accessible.”
The current price of hearing aids averages more than $5,000 per pair, and they are not typically covered by traditional Medicare or other insurers. Vice President Harris said the rule would reduce the cost of hearing aids by hundreds and even thousands of dollars.
“Every American has the right to receive affordable health care,” she said in a statement Tuesday. “Today our administration has taken another step forward in our fight to protect that right.”
A study published in Social Science and Medicine in 2019 found that the counties with the largest numbers of older adults with hearing loss often had fewer available audiologists, in part because the doctors tend to practice in younger, wealthier urban areas.
Stigma, lack of access and confusion about how to get the best health care often prevent people — especially older Americans — from taking care of their hearing health, said Barbara Kelley, executive director of the Hearing Loss Association of America. This option will benefit countless Americans who might need hearing assistance in perhaps restaurants or large family gatherings without necessarily seeking a hearing professional, she said.
“For years, we have worked for affordable and accessible hearing health care, and this is a huge step in getting people to pay attention to their hearing health sooner rather than later,” Kelley said. “And this just provides another avenue — a new avenue truly — for adults with mild to moderate hearing loss who can take a step on their own.”
Although about 38 million adults in the United States report hearing loss, few have tried the devices. Among adults over 70 with hearing loss, only one in three have ever worn one, according to data collected in the National Health Interview Survey.
The FDA’s move follows years of federal efforts to remove obstacles between patients and over-the-counter hearing aids. In 2015, the President’s Council of Advisors on Science and Technology under Barack Obama recommended that the FDA create a new category of “basic” hearing aids that could be purchased without a prescription or a doctor’s visit. Two years later, President Donald Trump signed the Over-the-Counter Hearing Aid Act of 2017, which gave the FDA three years to enact the new rules.
The FDA missed that 2020 deadline, but President Biden renewed pressure in July 2021 when he signed an executive order that set a November deadline for a new proposed rule from the federal agency.
[ad_2]
Source link